Mass Defect And Binding Energy Worksheet. Mass defect is the difference between the predicted mass and the actual mass of an atom's The binding energy of a system can appear as extra mass, which accounts for this difference. Worksheet will open in a new window.

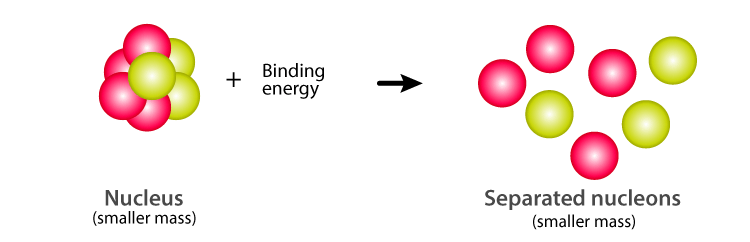
Mass defect is the difference between the predicted mass and the actual mass of an atom's The binding energy of a system can appear as extra mass, which accounts for this difference.
Divide this value by the number of.
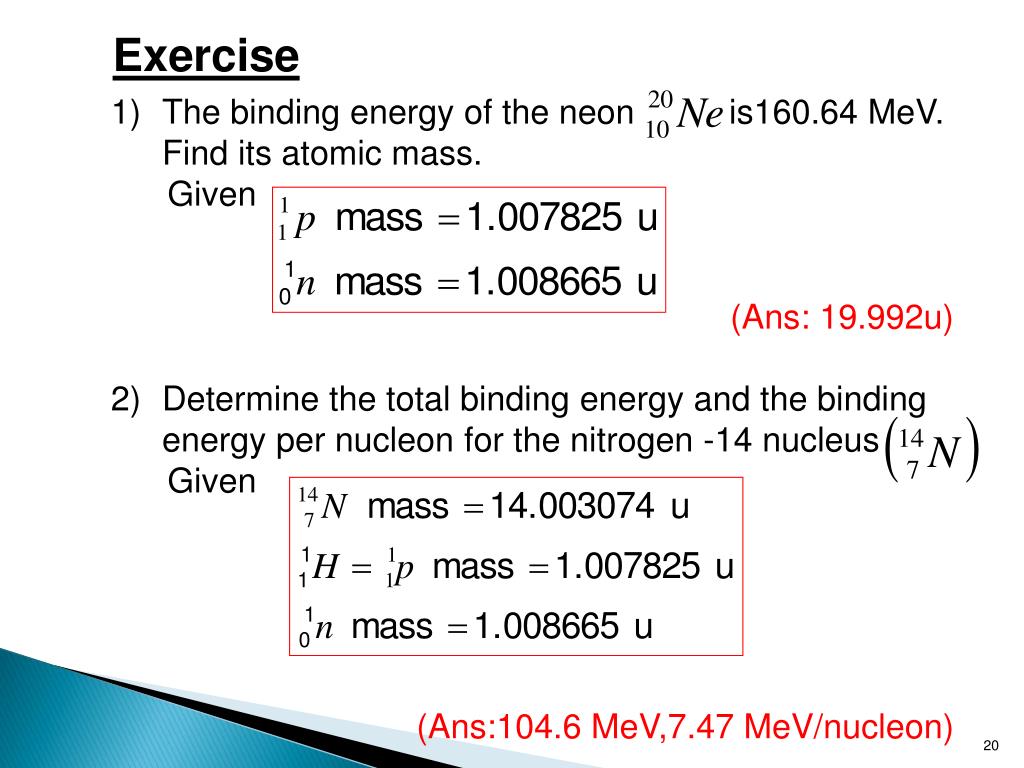
Worksheet will open in a new window. In this lesson we'll look in more detail at the amount of energy released when nuclei become Negative potential energy is energy you didn't have to put in. To compare the stability of different nuclides: Work out the nucleus' mass defect (compare its overall mass to that of its constituent parts) Use E=mc to.