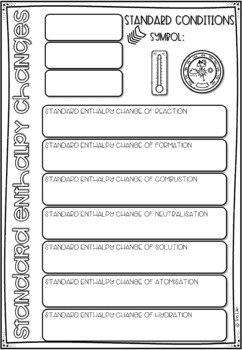
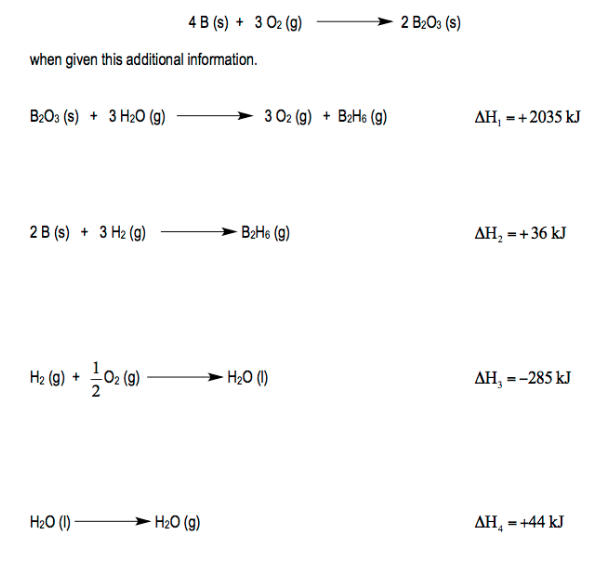
Enthalpy Change Worksheet. Enthalpy changes can be measured experimentally by measuring the temperature change as a reaction proceeds. Explaining Hess's Law Hess's Law is saying that if you convert reactants A into products B, the overall enthalpy change will be exactly the same whether you do it in one step or two steps or however many steps.

A reaction is performed inside a constant pressure calorimeter that consists of an insulated container with water in it.
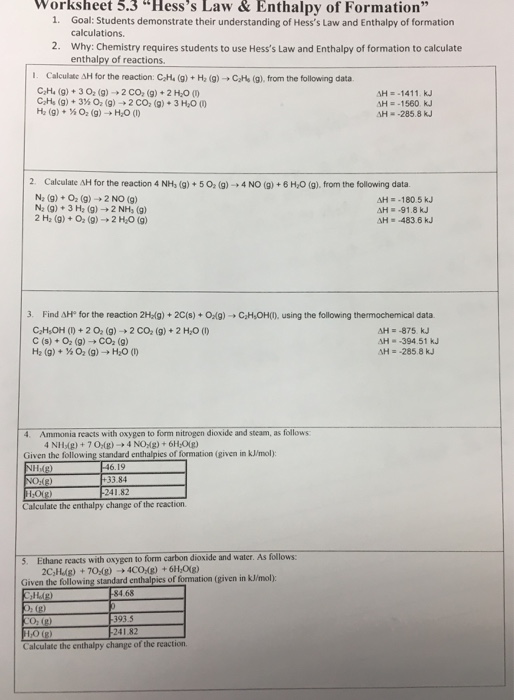
The calculation of ΔH for these equations from thermochemical data is an important skill.
The enthalpy change for a reaction is independent of path. Measuring Enthalpy Changes Worksheet As you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. The enthalpy change for a reaction is the heat produced or absorbed by a reaction at constant pressure (qp).