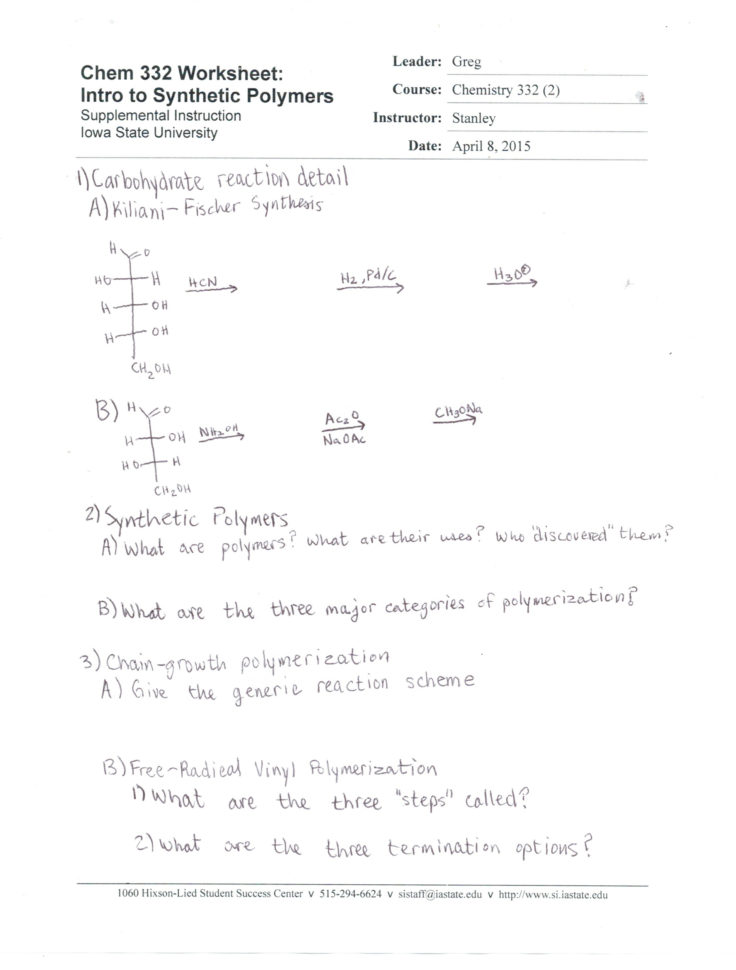
Synthesis And Decomposition Reactions Worksheet. These are the rules for synthesis and decomposition reactions from "The Ultimate Chemical Equations Handbook." decompose into metallic oxides and carbon dioxide A sample of magnesium carbonate is heated. Download synthesis and decomposition worksheet answers for FREE.
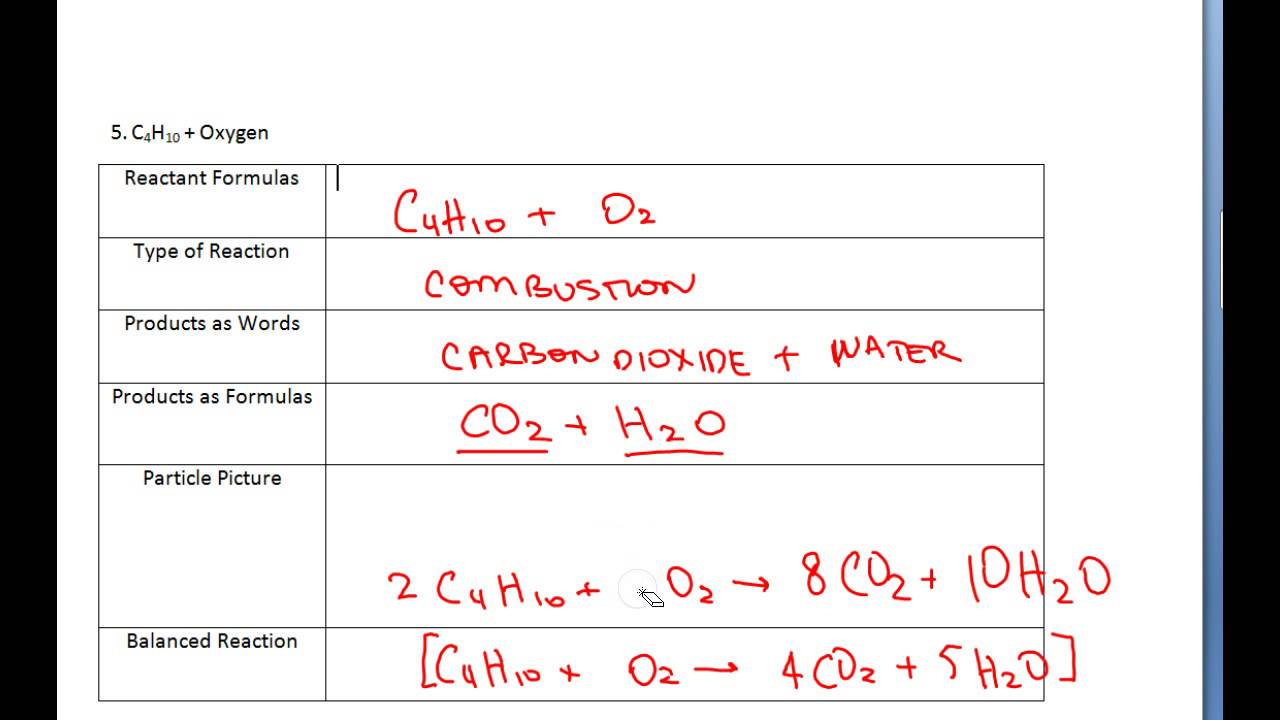
In decomposition reactions, one compound will break down into two or more parts. barium carbonate.
It is defined as the reaction in which a single compound splits into two or more simple substances under suitable Decomposition of potassium chlorate:When heated strongly, potassium chlorate decomposes into potassium chloride and oxygen.
Go through the reactions on this worksheet and identify any that are decomposition or synthesis reactions ONLY. A decomposition reaction starts from a single substance and produces more than one substance; that is, it decomposes. The questions are grouped into combustion, synthesis, and decomposition reactions.